Network Meta-analyses
What is a Network Meta-analysis?
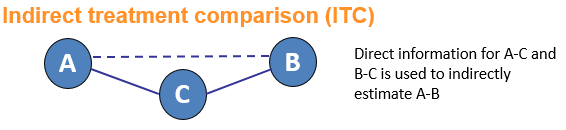
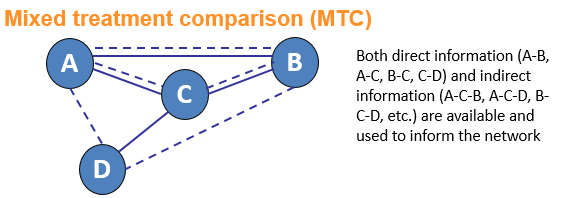
Network meta-analysis is an umbrella term which can be used to describe indirect and mixed treatment comparisons (ITC/MTC). They are statistical tools for assessing, for example, the comparative efficacy and safety of interventions when:
- There is no direct evidence between the interventions of interest
- There is insufficient direct evidence or
- There are more than two interventions that need to be compared
NMA methods are developing rapidly and are already being widely used in health care to inform decisions which require an understanding of the comparative effectiveness of different interventions.
Why conduct an NMA?
HTA submission
- NMAs are now accepted as part of health technology assessments in many countries including England (NICE), France (Haute Autorité de Santé), Canada (Canadian Agency for Drugs and Technologies in Health) and Australia (Pharmaceutical Benefits Advisory Committee).
- We can provide all of the documentation required by the specific HTA agency and can help you to populate the relevant section of the submission template.
Inform an economic model
- At YHEC, we have experience of building and adapting economic models for hundreds of health care technologies. The outputs of NMA can be used to inform the model inputs.
Produce a publication
- We can support you to carry out a systematic review and conduct NMA in line with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) NMA reporting guidelines.
Inform future trials
- We can help you interpret the results of your trial within the context of comparator trials, or to assess the landscape of comparator trials and inform plans for future clinical trials by assessing where your trial could fit in the evidence network.
How can we help you?
As with a standard pairwise meta-analysis, the validity of an NMA depends on:
- The adequacy of the evidence base
- The similarity of the trials
These issues underpin the focus of the systematic review and feasibility assessment of whether an NMA is possible. Formal feasibility assessment of the similarity of trials is crucial to a high quality and robust network.
We can work with you right through from question development to conducting the NMA or we can review and critique existing or published NMA.
 Local Health and Public Sector Organisations
Local Health and Public Sector Organisations